How To Find Nonbonding Electrons
Lewis Structures
Writing Lewis Structures by Trial and Error
The Lewis structure of a compound can be generated by trial and mistake. We get-go by writing symbols that contain the correct number of valence electrons for the atoms in the molecule. We and so combine electrons to grade covalent bonds until we come with a Lewis structure in which all of the elements (with the exception of the hydrogen atoms) have an octet of valence electrons.
Example: Let's apply the trial and error approach to generating the Lewis construction of carbon dioxide, CO2. We start by determining the number of valence electrons on each atom from the electron configurations of the elements. Carbon has four valence electrons, and oxygen has six.
C: [He] 2due south two twop 2
O: [He] iidue south 2 2p 4
We can symbolize this information every bit shown at the top of the figure below. We now combine one electron from each atom to grade covalent bonds between the atoms. When this is done, each oxygen atom has a full of seven valence electrons and the carbon atom has a total of half-dozen valence electrons. Because none of these atoms accept an octet of valence electrons, nosotros combine another electron on each atom to course ii more bonds. The result is a Lewis structure in which each atom has an octet of valence electrons.

![]()
A Step-Past-Step Arroyo To Writing Lewis Structures
The trial-and-error method for writing Lewis structures tin can exist fourth dimension consuming. For all only the simplest molecules, the post-obit step-by-step process is faster.
Step i: Determine the total number of valence electrons.
Step ii: Write the skeleton structure of the molecule.
Stride three: Use two valence electrons to form each bond in the skeleton construction.
Step four: Try to satisfy the octets of the atoms past distributing the remaining valence electrons as nonbonding electrons.
The outset stride in this process involves calculating the number of valence electrons in the molecule or ion. For a neutral molecule this is cypher more than the sum of the valence electrons on each cantlet. If the molecule carries an electric charge, nosotros add one electron for each negative accuse or subtract an electron for each positive charge.
Instance: Let's determine the number of valence electrons in the chlorate (ClOiii -) ion.
A chlorine cantlet (Group VIIA) has seven valence electrons and each oxygen atom (Group VIA) has six valence electrons. Considering the chlorate ion has a accuse of -one, this ion contains one more electron than a neutral ClOiii molecule. Thus, the ClO3 - ion has a full of 26 valence electrons.
ClO3 -: 7 + three(half-dozen) + 1 = 26
The second pace in this procedure involves deciding which atoms in the molecule are connected by covalent bonds. The formula of the compound often provides a hint equally to the skeleton construction. The formula for the chlorate ion, for example, suggests the following skeleton structure.
![]()
The third step assumes that the skeleton construction of the molecule is held together past covalent bonds. The valence electrons are therefore divided into 2 categories: bonding electrons and nonbonding electrons. Because it takes two electrons to form a covalent bond, we can summate the number of nonbonding electrons in the molecule past subtracting two electrons from the total number of valence electrons for each bond in the skeleton structure.
There are three covalent bonds in the almost reasonable skeleton structure for the chlorate ion. Every bit a result, half-dozen of the 26 valence electrons must exist used as bonding electrons. This leaves xx nonbonding electrons in the valence shell.
| 26 valence electrons |
| - 6 bonding electrons |
| 20 nonbonding electrons |
The nonbonding valence electrons are now used to satisfy the octets of the atoms in the molecule. Each oxygen cantlet in the ClOiii - ion already has 2 electrons ![]() the electrons in the Cl-O covalent bail. Considering each oxygen atom needs six nonbonding electrons to satisfy its octet, it takes 18 nonbonding electrons to satisfy the three oxygen atoms. This leaves one pair of nonbonding electrons, which tin exist used to make full the octet of the cardinal cantlet.
the electrons in the Cl-O covalent bail. Considering each oxygen atom needs six nonbonding electrons to satisfy its octet, it takes 18 nonbonding electrons to satisfy the three oxygen atoms. This leaves one pair of nonbonding electrons, which tin exist used to make full the octet of the cardinal cantlet.

![]()
Drawing Skeleton Structures
The most difficult part of the iv-stride process in the previous section is writing the skeleton structure of the molecule. Equally a general dominion, the less electronegative chemical element is at the center of the molecule.
Example: The formulas of thionyl chloride (SOCltwo) and sulfuryl chloride (SO2Cl2) tin be translated into the following skeleton structures.

It is also useful to recognize that the formulas for circuitous molecules are frequently written in a style that hints at the skeleton construction of the molecule.
Example: Dimethyl ether is oft written as CHiiiOCH3, which translates into the post-obit skeleton structure.

Finally, information technology is useful to recognize that many compounds that are acids incorporate O-H bonds.
Example: The formula of acetic acrid is oftentimes written every bit CH3COiiH, because this molecule contains the following skeleton construction.

![]()
Molecules that Contain Too Many or Not Enough Electrons
As well Few Electrons
Occasionally nosotros encounter a molecule that doesn't seem to have plenty valence electrons. If we tin't go a satisfactory Lewis structure past sharing a single pair of electrons, it may be possible to achieve this goal by sharing two or even three pairs of electrons.
Instance: Consider formaldehyde (HtwoCO) which contains 12 valence electrons.
H2CO: 2(1) + 4 + 6 = 12
The formula of this molecule suggests the following skeleton construction.
![]()
There are three covalent bonds in this skeleton structure, which means that half dozen valence electrons must be used every bit bonding electrons. This leaves six nonbonding electrons. It is impossible, however, to satisfy the octets of the atoms in this molecule with only six nonbonding electrons. When the nonbonding electrons are used to satisfy the octet of the oxygen atom, the carbon atom has a total of only six valence electrons.
We therefore assume that the carbon and oxygen atoms share 2 pairs of electrons. There are at present four bonds in the skeleton structure, which leaves only four nonbonding electrons. This is enough, nonetheless, to satisfy the octets of the carbon and oxygen atoms.

Every once in a while, nosotros encounter a molecule for which it is impossible to write a satisfactory Lewis structure.
Instance: Consider boron trifluoride (BFiii) which contains 24 valence electrons.
BF3: 3 + three(7) = 24
There are three covalent bonds in the near reasonable skeleton structure for the molecule. Because it takes half-dozen electrons to form the skeleton construction, there are 18 nonbonding valence electrons. Each fluorine atom needs half dozen nonbonding electrons to satisfy its octet. Thus, all of the nonbonding electrons are consumed by the three fluorine atoms. Every bit a result, we run out of electrons while the boron atom has but six valence electrons.

The elements that class stiff double or triple bonds are C, North, O, P, and South. Because neither boron nor fluorine falls in this category, we have to stop with what appears to be an unsatisfactory Lewis structure.
Too Many Electrons
It is likewise possible to encounter a molecule that seems to have too many valence electrons. When that happens, nosotros expand the valence crush of the central cantlet.
Example: Consider the Lewis construction for sulfur tetrafluoride (SF4) which contains 34 valence electrons.
SF4: half dozen + iv(7) = 34
At that place are four covalent bonds in the skeleton structure for SF4. Considering this requires using eight valence electrons to class the covalent bonds that agree the molecule together, there are 26 nonbonding valence electrons.
Each fluorine atom needs six nonbonding electrons to satisfy its octet. Because there are iv of these atoms, so we need 24 nonbonding electrons for this purpose. But there are 26 nonbonding electrons in this molecule. We have already satisfied the octets for all five atoms, and nosotros nonetheless have 1 more pair of valence electrons. We therefore expand the valence beat of the sulfur atom to agree more than eight electrons.

This raises an interesting question: How does the sulfur atom in SF4 hold 10 electrons in its valence shell? The electron configuration for a neutral sulfur cantlet seems to advise that information technology takes 8 electrons to fill the 3due south and 3p orbitals in the valence shell of this atom. But allow'southward await, once once more, at the selection rules for diminutive orbitals. According to these rules, the northward = 3 crush of orbitals contains 3southward, 3p, and 3d orbitals. Because the 3d orbitals on a neutral sulfur atom are all empty, one of these orbitals can be used to hold the actress pair of electrons on the sulfur cantlet in SFfour.
S: [Ne] 3s 2 threep 4 threed 0
![]()
Resonance Hybrids
Ii Lewis structures tin be written for sulfur dioxide.
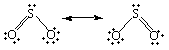
The but difference between these Lewis structures is the identity of the oxygen atom to which the double bond is formed. As a result, they must be equally satisfactory representations of the molecule.
Interestingly enough, neither of these structures is correct. The two Lewis structures suggest that 1 of the sulfur-oxygen bonds is stronger than the other. In that location is no divergence betwixt the length of the two bonds in SO2, however, which suggests that the two sulfur-oxygen bonds are equally strong.
When nosotros can write more than one satisfactory Lewis structure, the molecule is an average, or resonance hybrid, of these structures. The pregnant of the term resonance tin be best understood by an analogy. In music, the notes in a chord are oftentimes said to resonate ![]() they mix to give something that is more than than the sum of its parts. In a similar sense, the two Lewis structures for the SO2 molecule are in resonance. They mix to requite a hybrid that is more the sum of its components. The fact that And so2 is a resonance hybrid of two Lewis structures is indicated by writing a double-headed arrow betwixt these Lewis structures, as shown in the figure in a higher place.
they mix to give something that is more than than the sum of its parts. In a similar sense, the two Lewis structures for the SO2 molecule are in resonance. They mix to requite a hybrid that is more the sum of its components. The fact that And so2 is a resonance hybrid of two Lewis structures is indicated by writing a double-headed arrow betwixt these Lewis structures, as shown in the figure in a higher place.
![]()
Formal Accuse
Information technology is sometimes useful to calculate the formal accuse on each atom in a Lewis structure. The first step in this adding involves dividing the electrons in each covalent bail between the atoms that grade the bail. The number of valence electrons formally assigned to each atom is so compared with the number of valence electrons on a neutral cantlet of the element. If the cantlet has more valence electrons than a neutral cantlet, information technology is assumed to carry a formal negative accuse. If it has fewer valence electrons information technology is assigned a formal positive accuse.
The formula of the amino acrid known as glycine is often written as H3N+CH2COtwo -. Use the concept of formal charge to explicate the meaning of the positive and negative signs in the following Lewis construction.

Click here to check your answer to Practice Problem 5
![]()
How To Find Nonbonding Electrons,
Source: https://chemed.chem.purdue.edu/genchem/topicreview/bp/ch8/lewis.php
Posted by: bezansonthemon.blogspot.com


0 Response to "How To Find Nonbonding Electrons"
Post a Comment